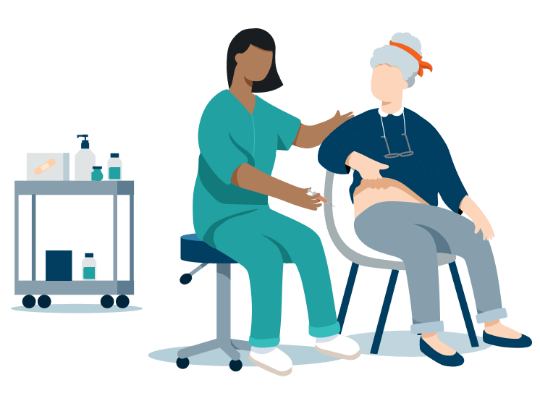
Discover OPDIVO Qvantig™, an immunotherapy treatment given by injection that's faster*
OPDIVO Qvantig is an alternative administrative option FDA approved to treat most cancer types approved for OPDIVO® (nivolumab).†

Discover OPDIVO Qvantig™, an immunotherapy treatment given by injection that's faster*
OPDIVO Qvantig is an alternative administrative option FDA approved to treat most cancer types approved for OPDIVO® (nivolumab).†
Actor portrayals.
*A 3-5 minute injection time of OPDIVO Qvantig compared to a 30-minute infusion time of OPDIVO. This does not account for all aspects of treatment. Does not include observation time. Actual clinic time may vary.
†OPDIVO Qvantig is not indicated for co-administration with YERVOY® (ipilimumab) or in pediatric patients.
OPDIVO Qvantig is an immunotherapy treatment that is delivered differently than OPDIVO IV. Both work with the immune system in the same way, but OPDIVO Qvantig doesn’t need to be administered at an infusion center and may be given at a doctor’s office or a site closer to home. Ask your doctor if a subcutaneous injection may be right for you.
Given as an intravenous infusion
directly into a vein using a needle or tube.
Takes 30 minutes
This refers only to the time it takes to receive treatment and does not account for all aspects of treatment. Actual time at the clinic may vary.
May require a port
A port is a small device that is surgically implanted and connected to a vein. When medication is administered, a needle connected to an IV tube is inserted into the port.
Given as an injection
by a healthcare professional under the skin in the stomach area (abdomen) or thigh area.
Takes 3-5 minutes
This refers only to the time it takes to give the injection and does not account for all aspects of treatment. Actual time at the clinic may vary.
No port needed
Since medication is injected under the skin and no access to a vein is required, a port is not necessary.
Alone or with other therapies, OPDIVO Qvantig is FDA approved to treat many types of cancer. Please see full list of eligible cancer types below.
OPDIVO Qvantig showed similar results and side effects to infusions of OPDIVO IV in a clinical trial
495 PEOPLE WITH PREVIOUSLY TREATED KIDNEY CANCER WERE STUDIED.
NO MAJOR DIFFERENCES WERE OBSERVED in the amount of medicine that went into the bloodstream between OPDIVO Qvantig and OPDIVO IV
†OPDIVO Qvantig injections are given by a healthcare provider in 3-5 minutes. This does not account for all aspects of treatment. Actual clinic time may vary.
See full indication list in the carousel at the top of the page.
OPDIVO Qvantig is approved alone or in combination with other cancer medication for adults with the following cancers.
Non-small cell lung cancer:
Early-stage non-small cell lung cancer (NSCLC) before surgery
Early-stage non-small cell lung cancer (NSCLC) before and after surgery
Advanced kidney cancer:
Previously untreated kidney cancer that has spread (advanced renal cell carcinoma)
Previously treated kidney cancer that has spread (advanced renal cell carcinoma)
Gastroesophageal cancer:
Bladder cancer:
Bladder or urinary tract cancer after surgery
Advanced cancer in the bladder or urinary tract
Previously treated advanced bladder or urinary tract cancer
Head and neck squamous cell cancer:
Previously treated squamous cell cancer of the head and neck
Melanoma:
OPDIVO Qvantig is approved alone for adults and children 12 and older with the following cancers.
Melanoma that has spread or cannot be removed by surgery (advanced melanoma)
Stage 2B or stage 2C melanoma after it has been completely removed by surgery
Stage 3 or stage 4 melanoma after it has been completely removed by surgery
Find out if OPDIVO Qvantig is right for you with answers to common questions people have about OPDIVO Qvantig.
This information is not a substitute for talking to your healthcare provider because they are the best source of information about your condition.
*Please note, treatment is delivered in 3-5 minutes. This refers only to the time it takes to give the injection and does not account for all aspects of treatment. Actual time at the clinic may vary.
†OPDIVO Qvantig is not indicated for co-administration with YERVOY® (ipilimumab).

Learn more about the complimentary OPDIVO with You support program
Track your symptoms and other important information so that you can proactively manage potential side effects with your doctor
See which cancer types can be treated with OPDIVO Qvantig and possible side effects