Clinical trial results for
previously treated stage 4 recurrent advanced
non-small cell
lung cancer (NSCLC)
For adults with previously treated advanced non-small cell lung cancer (NSCLC)

Clinical trial results for
previously treated stage 4 recurrent advanced
non-small cell
lung cancer (NSCLC)
For adults with previously treated advanced non-small cell lung cancer (NSCLC)
Actor portrayals.
OPDIVO and OPDIVO Qvantig are not approved for people
younger than 18 years of age.
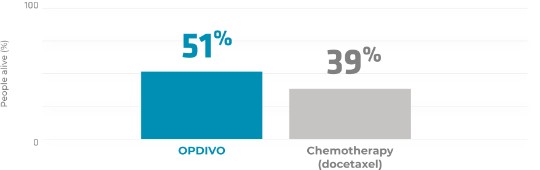
In a clinical trial of 582 people with previously treated non-squamous stage 4 recurrent NSCLC that had spread or grown after treatment with platinum-based chemotherapy, 292 people were given OPDIVO and 290 people were given chemotherapy (docetaxel).
Half the people were alive
Half the people were alive
In the primary analysis with a follow-up at 1 year, people given OPDIVO had a 27% lower risk of dying than those given chemotherapy.
OPDIVO will not work for everyone. Individual results may vary.
In a clinical trial of 272 people with squamous NSCLC that had spread or grown after treatment with platinum-based chemotherapy, 135 people were given OPDIVO and 137 people were given chemotherapy (docetaxel).
Half the people were alive
Half the people were alive
In the primary analysis with a follow-up at 1 year, people given OPDIVO had a 41% lower risk of dying than those given chemotherapy.
OPDIVO will not work for everyone. Individual results may vary.
It is not known if OPDIVO Qvantig is safe and effective in children. OPDIVO Qvantig cannot be used in combination with ipilimumab.
*A 3-5 minute injection time of OPDIVO Qvantig compared to a 30-minute infusion time of OPDIVO. This does not account for all aspects of treatment. Does not include observation time. Actual clinic time may vary.
John is a husband, father of two, and a passionate golfer. Learn more about his experience with OPDIVO for his previously treated NSCLC.

See treatment plans and what to expect from an intravenous (IV) infusion or under-the-skin injection (subcutaneous injection)
For adults with previously treated advanced non-small cell lung cancer
As an intravenous infusion
OPDIVO® (nivolumab) is a prescription medicine used to treat adults with a type of advanced stage lung cancer (called non-small cell lung cancer) that has spread and you have received chemotherapy that contains platinum, and it did not work or is no longer working. If your tumor has an abnormal EGFR or ALK gene, you should have also received an EGFR or ALK inhibitor medicine and it did not work or is no longer working.
It is not known if OPDIVO is safe and effective in children younger than 12 years of age with melanoma or MSI-H or dMMR metastatic colorectal cancer.
It is not known if OPDIVO is safe and effective in children for the treatment of any other cancers.
As a subcutaneous injection
OPDIVO Qvantig™ (nivolumab + hyaluronidase-nvhy) is a prescription medicine used alone to treat adults with a type of advanced stage lung cancer (called non-small cell lung cancer) that has spread and you have received chemotherapy that contains platinum, and it did not work or is no longer working. If your tumor has an abnormal EGFR or ALK gene, you should have also received a FDA-approved therapy for tumors with these abnormal genes, and it did not work or is no longer working.
It is not known if OPDIVO Qvantig is safe and effective in children.